
Experimental demonstration. “Alchemist Sedziwoj” by Jan Matejko, oil on canvas, public domain
What is the first image conjured up in your mind by the word “alchemy”? Influenced by popular culture, it is tempting to picture: somewhere in Renaissance Europe, in a dark dungeon, groups of alchemists fiddling with crucibles over some “book of secrets,” on a quest for the philosophers’ stone, and in pursuit of “transmutation” (i.e. making gold). The exclusivity and secrecy behind alchemy seem to suggest alchemy was the opposite of enlightenment, progress, and modern science. However, there are increasing numbers of studies indicating otherwise (e.g. Martinón-Torres and Rehren, 2005; Martinón-Torres, 2012; Mongiatti, 2009; Veronesi et al., 2021). The practice of alchemy could be more scientific, methodical, and industrial than people have previously imagined. In fact, before 1753, the words “chemistry” and “alchemy” were synonymous (Martinón-Torres and Rehren, 2005).
The principal aim of this post is to demonstrate that the boundary between alchemy and chemistry was much more fluid (e.g. Martinón-Torres and Rehren, 2005). It is also my intention to show how alchemy was strongly intertwined with contemporary societies. In sixteenth-century Europe, there was a peak in metallurgical advancements (such as mining or minting) along with the publishing of technical treatises and alchemical text (Long, 1991). Against this background, I will address the scholarly discussion on the dichotomy of authorship between alchemical and technical writing (i.e. secrecy vs. openness). Next, I will discuss a case study on an (alchemical) laboratory in seventeenth-century England.
From a methodological perspective, this article addresses the case study predominately through material analysis. I would also like to emphasize how archaeological science can add a unique point of view to the history of science (Martinón-Torres, 2011; Martinón-Torres and Rehren, 2005).
Setting the Scene: In the 16th Century Europe
Sixteenth-century Europe witnessed the bloom of metallurgical advancements. This phenomenon was driven by political and economic factors, but also partly by the lack of silver. Silver was an important metal since it was the basis of monetary exchange before paper money was invented. The authorities recognized the importance of using technical inventions to increase silver production. Many surveyors, mining consultants, metallurgists, and other experts were sponsored to develop or improve technology (Long, 1991, 2001; Nef, 1987, Nummedal, 2007). In this climate, in addition to metallurgists, alchemists were also valued by the authorities because of their ability to identify and exploit minerals (Nummedal, 2007: 80-81; Jütte, 2015: 104). Hence, it was common at the time for patrons to support big-scale alchemical laboratories. In fact, documents show that “liquidation” a complicated technology to extract silver from copper/silver ores was first suggested by an alchemist to the Duke of Saxony, (Nummedal, 2007: 89) indicating that alchemy made contributions to what we would consider “science” today.
Many technical writings were also published during this period. One of them is De re Metallica by Georgius Agricola (1566). De re Metallica was even brought to Late Ming China by missionaries and translated into Chinese in the late 17th century (Jixing, 1991). An English translation by Hoover and Hoover was published in 1912. These Hoovers were also to become the president and first lady of the USA. Agricola was a humanist and a doctor but also had a life-long connection with skilled artisans and practitioners. He was a doctor taking care of miners at Jáchymov, Czech Republic (1527- 1531), where he was based for his metallurgical studies and observations (Majer, 1994: 92). Despite his short stay at Jáchymov, it was during the time when the local mining industry was at its peak (ibid.). He wrote De re Metallica based on observations gained from his time in Jáchymov and during his travels (Long, 2001). As one of the most influential metallurgical treatises, De re Metallica had detailed illustrations and covered a wide range of topics (such as finding ores and veins, digging tunnels, tools used for mining to assaying and smelting various types of ores). It is also interesting to note that Agricola was an advocator on the openness of knowledge. Agricola condemned the alchemists for not being transparent on the authorship of the writing and the lack of efficacy on their results (Long, 1991, 2001).
Another important technical writing on metallurgy is Treatise on Ores and Assaying (1574) by Lazarus Ercker. Ercker was a practitioner of assaying and minting. He was a control assayer at Royal Mint, Kutná Hora, Czech Republic. Ercker also criticized alchemical writing that failed to produce practical results (Long, 1991).
It is undeniable that alchemy writing had its own traditions, compared with the writing of mining and metallurgy. The authorship tended to be hidden and the terminology was obscure and it was hard to reproduce the same results. The transmission of alchemical knowledge was usually within apprenticeships. Though the writing traditions might be in opposition, it does not mean that metallurgists/practitioners and alchemists did not learn from each other. Despite Agricola’s dislike of alchemists, he mentioned they were the inventors of a method that separated gold and silver with nitric acid (Hoover and Hoover, 1950). Ercker also mentioned in his book “assaying is a very excellent, ancient, and useful art, which, like all other arts that work with fire, originated very long ago as an outgrowth of alchemy” (Sisco and Smith 1951, 9). In the Oberstockstall laboratory, a medieval alchemical/chemical laboratory, Agricola’s publications were present in the book inventory (Martinón-Torres and Rehren, 2005).
Alchemist or Chemist? Case Study Old Ashmolean Laboratory, Oxford
The Ashmolean Museum was opened in 1683 in Oxford. The building today is the Museum of the History of Science. Other than artifact collections, the museum also included a library, a lecture theatre and a state-of-the-art laboratory (Bennet et al, 2000 from Veronesi et al., 2021). As part of the University of Oxford, the museum was where chemistry was officially taught in England. The first keeper of the museum, who was also the first chemistry professor of the university was Robert Plot (Martinón-Torres, 2012; Veronesi et al., 2021), who had known alchemical interests (see Taylor, 1949). Interestingly, though it was a high-profile chemical laboratory, the written sources on what Plot and his assistant were experimenting with in Ashmolean are relatively scarce.
Excavations of the laboratory in 1999 recovered ceramics associated with experiments. The technique of SEM-EDS (scanning electron microscopy with energy-dispersive X-ray spectroscopy) was employed to analyze these fragments. This technique allows users to have a clear image of the materials’ microstructure under high-resolution magnification (SEM) and the chemical composition (EDS). Both lines of information are useful in interpreting the manufacture and usage of the materials. For example, in the figure below (Veronesi et al., 2021), there are two SEM micrographs that show the ceramic matrix from two samples. On the left, Q are quartz grains and G are grog inclusion (Note: in archaeology, grog usually refers to any type of crushed fired ceramic.). On the right, the black phases are flakes of graphite. These identifications were achieved through EDS. The information is valuable because it shows the difference in the production of ceramic. The operational choices behind these differences in manufacture could derive from different purposes of ceramic usage, available materials, or local tradition.
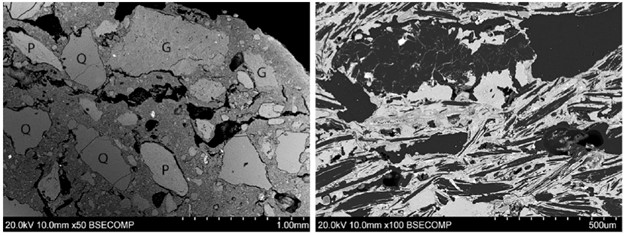
SEM-EDS (scanning electron microscopy with energy dispersive X-ray spectroscopy) scans of two samples, (Veronesi et al., 2021)
The analysis of SEM-EDS has shown that these ceramic fragments were used for wide ranges of activities, from the distillation of zinc, the development/production of new types of glass (e.g. lead crystal glass) to the alchemy related ‘metallic transmutation’ i.e. extraction of precious metals (for the analytical details see Veronesi et al., 2021). The experimentation on lead crystal glass drew particular interest in how the chemistry (or alchemical?) laboratory connected with contemporary societies. Lead crystal glasses were invented in the late 17th century. Because of its material properties, the lead-based glass was more transparent and had a higher grade of purity compared with other types of glass (e.g. lime-based) at that time. It is also more easily made into high-quality glassware for wealthy customers (Martinón-Torres, 2012). Needless to say, the knowledge of lead crystal glass production was financially valuable. Hence, the lack of direct records of Plot’s experimentation and other indirect written sources indicate that Plot was experimenting with lead glass. The silence was to keep the trade secret (Martinón-Torres, 2012; Veronesi et al., 2021).
Conclusions
Does it matter if the Old Ashmolean laboratory is an alchemical or chemical laboratory? Maybe the distinction is not that important. The material analysis and the written sources presented above indicate that the boundary between the alchemists and the practitioners/chemists was more fluid and the relationship was more complex. Metallurgical knowledge did not stay in one realm. The transmission of knowledge was open and vibrant. Alchemists were also active in contemporary societies. They were valued by the authorities for their knowledge.
This post also shows how archaeological science contributes to the study of the history of science. Archaeological science provides different lines of evidence that can explain or compensate the written sources. As an archaeological practitioner myself, I do not want to destroy those “sexy” images of the past (Yes, I still like to imagine alchemists were like wizards in Harry Potter… cooking up magic in the dark basement). Despite that, I hope this post not only shows the complexity of the alchemists/chemists identification in the past but also provides some thoughts on the dissemination of knowledge in the time when open access to information and modern chemistry was in its infancy.
References
Bennet, J.A., Johnston, S.A. and Simcock, A.V. (2000) Solomon’s House in Oxford: New finds from the First Museum. Oxford: Museum of the History of Science.
Hoover, H. C. and Hoover, L. H. (1950) De Re Metallica, Georgius Agricola, Translated from the first Latin edition of 1556, New York: Dover Publications Inc.
Hsu, Y. (2018) 還原歷史的不可或缺-煉金術考古[the archaeology on alchemy ] , Science Monthly [科學月刊], 6, 430-433.
Jixing, P. (1991) The Spread of Georgius Agricola’s “De Re Metallica” in Late Ming China, T’oung Pao, 77, 108-118
Jütte, D. (2015) The Age of Secrecy: Jews, Christians, and the economy of Secrets, 1400-1800, New Heaven: Yale University Press.
Long, P.O. (1991) The Openness of Knowledge: An Ideal and Its Context in 16th-Century Writings on Mining and Metallurgy, Technology and Culture, 32, 318-355.
Long, P.O. (2001) Openness, Secrecy, Authorship. Baltimore: The John Hopkins University Press.
Majer, J. (1994) Ore mining and the town of St. Joachimsthal (Jáchymov) at the Time of Georgius Agricola, GeoJournal, 32, 91-100.
Martinón-Torres, M. (2011) Some recent developments in the historiography of alchemy, Ambix, 58, 215–237.
Martinón-Torres, M. (2012) Inside Solomon’s house: an archaeological study of the Old Ashmolean Chymical Laboratory in Oxford, Ambix, 59, 22–48.
Martinón-Torres, M. and Rehren, T. (2005) Alchemy, chemistry and metallurgy in Renaissance Europe: a wider context for fire-assay remains, Historical Metallurgy, 39(1), 14-28.
Mongiatti, A. (2009) Assaying and Smelting Noble Metal in 16th Century Austria, PhD thesis. University of London.
Nef, J. (1987) Mining and Metallurgy in Medieval Civilisation. In: Miller, E., Postan, C. and Postan, M.M. (eds.) The Cambridge Economic History of Europe from the Decline of the Roman Empire, Cambridge: Cambridge University Press.
Nummedal, T. (2007) Alchemy and Authority in the Holy Roman Empire, Chicago: University of Chicago Press.
Sisco, A.G. and Smith, C.S. (1951) Lazarus Ercker’s Treatise on Ores and Assaying, Translated from the German edition of 1580, Chicago: The University of Chicago Press.
Szabadváry, F. (1966). History of Analytical Chemistry. London: Pergamon Press.
Taylor, S. (1949) Alchemical Papers of Dr Robert Plot, Ambix, 4, 67–76
Veronesi, U., Rehren, T. and Martinón-Torres, M. (2021) The philosophers and the crucibles. New data on the 17th–18th century remains from the Old Ashmolean laboratory, Oxford. Journal of Archaeological Science: Reports , 35 , Article 102684.
